Simeon Rossmann
Forsker
Biografi
Jeg forsker på forskjellige anvendte aspekter innenfor plantepatologi. Jeg har jobbet mye med Pectobacteriaceae, som forårsaker bløtråte og stengelråte i potet og ulike andre sykdommer i mange andre vertsplanter. Jeg er spesielt interessert i insekter assosiert med disse bakteriene og andre plantepatogene bakterier i feltet og molekylære mekanismene bak disse forskjellige assosiasjonene.
I det siste var jeg fokusert på praktiske og bioinformatiske implementeringer av metabarkoding eller amplikonsekvensering som et verktøy for deteksjon og identifisering av plantepatogene sopp, Oomycota, nematoder, bakterier, og invaderende plante- og insektarter.
Jeg fikk BSc og MSc fra University of Tübingen, hvor jeg oppdaget min lidenskap for plantepatologi ved Center for Plant Molecular Biology (ZMBP). I løpet av doktorgradsperioden min (2015-2018) undersøkte jeg bløtråte i norske potet med prof. May Bente Brurberg (NIBIO / NMBU) som hovedveileder.
Sammendrag
Det er ikke registrert sammendrag
Forfattere
May Bente Brurberg Simeon Rossmann Erik Lysøe Monica Skogen Håvard Eikemo Paulina Paluchowska Mirella Ludwiczewska Sylwester Sobkowiak Marta Janiszewska Zhimin Yin Jadwiga SliwkaSammendrag
Det er ikke registrert sammendrag
Sammendrag
Prosjektet «Mer Norsk Løk» (NFR 2522642) kartla dyrkings- og lagringspraksis hos 12 norske løkprodusenter fra 2022 til 2025 for å forbedre kvaliteten og øke forbruket av norsk løk. Kartleggingen inkluderte agronomiske tiltak, værdata, avlingsmålinger, lager klimadata og kvalitetsanalyse av lagringsprøver, med fokus på skallkvalitet, lagringsstabilitet og råte, i tillegg inngikk en stor patogenkartlegging basert på 300+ prøver og millioner av sekvensdata. Vekstsesongen varierte i lengde og tørre forhold under bakketørking viste seg å være avgjørende for kvalitet. Snitt avling var 6 846 kg/daa, med størst andel løk i fraksjonen 65–85 mm, og lagringspraksis viste store forskjeller i tid og temperaturkontroll. Målte klimadata (temperatur og relativ luftfuktighet) på de 12 lagre viste noe varierende temperaturforhold på de ulike lagrene med tørketemperatur fra 14 til 20 °C og relativ luftfuktighet på 52-85 samt temperaturer i hoved lagringsperioden på 0 til ca. 3 °C og relativ luftfuktighet på 80-90%. Kvalitetsvurdering viste vekttap på 3,1–8,9 %, lite råte generelt, men enkelte lagre hadde over 30 % råte i 2023–2024, en sesong som var preget av mye nedbør og smitte med løkbladskimmel. Patogenanalysene identifiserte Botrytis, Fusarium, Serratia, Pseudomonas og Peronospora som mest fremtredende. Mikrofloraen var påvirket av vær, dyrker og tidspunkt. Groing innvendig økte etter pakking og var mest utbredt i Østfold og Vestfold, knyttet til høyere temperaturer i vekstsesongen.

Divisjon for bioteknologi og plantehelse
Genomisk kartlegging av sykdomsresistensgener i potet
Potettørråte er den mest ødeleggende sykdommen i norsk potetproduksjon og gir store avlings- og økonomiske tap. For å redusere pesticidbruk og sikre bærekraftig matproduksjon må nye sorter utvikles med varig genetisk resistens.
Divisjon for bioteknologi og plantehelse
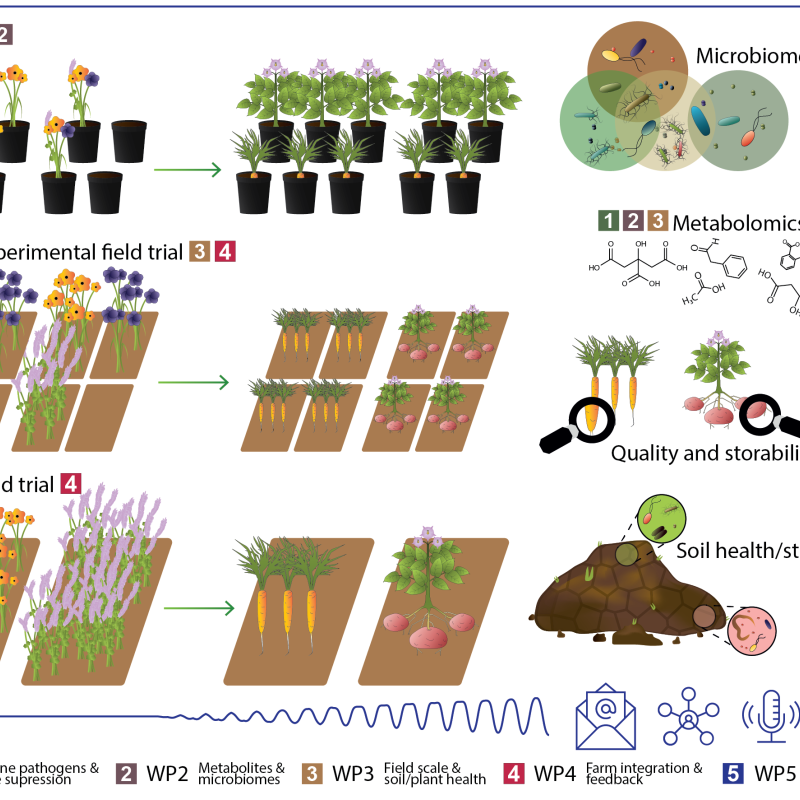
Cropdrive
Cropdrive aims to identify a selection of cover crops suitable for use in root vegetable and potato production with beneficial impacts on both soil and plant health, and greenhouse gas exchange.
