Divisjon for miljø og naturressurser
BioSynGas

Slutt: des 2026
Start: juni 2021
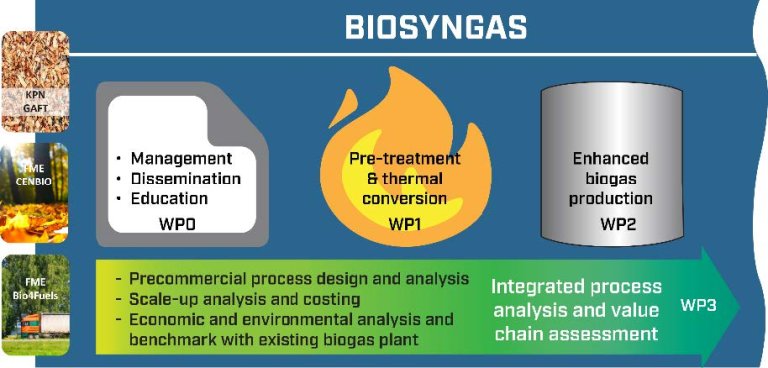
BioSynGas skal blant annet undersøke hvordan bioresten fra biogassproduksjon kan optimaliseres for videre bruk. I prosjektet skal det brukes biprodukter etter termisk behandling, som syngas og faste og flytende hydrotermiske produkter. Disse produktene har potensial til å gi et biogassanlegg økt kapasitet.
| Eksternt prosjektnettsted | BioSynGas prosjektnettside |
| Start- og sluttdato | 01.06.2021 - 31.12.2026 |
| Prosjektleder | Roger Khalil (SINTEF) |
| Prosjektansvarlig, NIBIO | Lu Feng |
| Divisjon | Divisjon for miljø og naturressurser |
| Avdeling | Bioressurser og kretsløpsteknologi |
| Samarbeidspartnere | SINTEF, NIBIO, NTNU, NMBU, Zhejiang University of Technology, Oslo Kommune, Bergen Kommune, Fredrikstad Vann Avløp og Renovasjonsforetak, Lindum AS, Vestfjorden Avløpsselskap, Hadeland og Ringerike Avfallsselskap AS, Antec Biogas AS |
| Finansieringskilde | Forskningsrådet |
Bioresten som er igjen etter at organisk avfall har blitt konvertert til biometan, inneholder mange verdifulle næringsstoffer. Den kan imidlertid inneholde mikro-/makroplast og tungmetaller, noe som er utfordrende med tanke på videre bruk. BioSynGas tar sikte på å løse disse utfordringene ved å behandle bioresten termisk.
Ved å integrere et termisk behandlingstrinn i eksisterende biogassanlegg, kan vi potensielt øke biometanproduksjonen og samtidig forbedre prosesseffektiviteten. En slik integrering vil også føre til at vi vil kunne behandle flere avfallsfraksjoner, noe som bidrar til økt fleksibilitet og økt biogassproduksjon.
BioSynGas vil også jobbe med prosessutvikling og modellering der integrasjonen optimaliseres. Viktige parametere som virkningsgrad, miljøbelastning og lønnsomhet vil bli kartlagt og sammenlignet med eksisterende frittstående løsninger.
BioSynGas er et samarbeid mellom SINTEF Energi (prosjekteier) og NIBIO og inkluderer to stipendiater knyttet til NTNU og NMBU. Zhejiang University of Technology, et anerkjent universitet i Kina, vil også bidra med aktivitet knyttet til hydrotermisk gassifisering. HRA, REG, VEAS, FREVAR, Lindum, Antec Biogas og Bergen Kommune er industripartnere.
NIBIOs rolle
NIBIO har ansvar for BioSynGas arbeidspakke 2 som går ut på å optimalisere biogassproduksjonen. Planen er å utføre forsøk for å maksimere produksjonen av biogass og fordøye kvaliteten ved bruk av biokull, syngas og hydrotermiske væsker og faste produkter.
For å oppnå dette, vil flere reaktorkonfigurasjoner bli brukt, inkludert batch- og kontinuerlig driftsstudier (ved bruk av for eksempel CSTR- og UASB-konfigurasjoner).
Én av forventningene til BioSynGas er at syngas kan forbedre og øke biometanproduksjonen i biogassreaktorer.
Publikasjoner i prosjektet
Forfattere
Begüm Bilgiç Judit Sandquist Svein Jarle Horn Lu Feng Cecilie Græsholt Asmira Delic Roger Antoine Khalil Michal SposobSammendrag
Digestate, a key byproduct of anaerobic digestion (AD), holds residual methane potential (RMP) that must be stabilized or recovered to prevent greenhouse gas emissions after field use. Thermal hydrolysis (TH), typically a pretreatment for AD, improves biogas production. This study assesses RMP in digestates from food waste (FW) and sewage sludge (SS) biogas plants, treated with TH at 160 and 190 °C. For the liquid fraction, FW digestate at 160 °C yielded 1.5 times more methane than untreated digestate, while SS digestate showed a threefold increase. The solid fraction of FW digestate at 160 °C had 1.4 times higher methane yield than untreated, but SS digestate produced less methane after TH. Adding sulfuric acid after TH increased phosphate release but reduced methane production in both digestates. Overall, TH as a post-treatment enhanced organic content release into the liquid fraction, enhancing methane yield, while acid addition improved phosphorus solubility, thereby enhancing digestate's nutrient value.
Sammendrag
Upgrading biogas to biomethane could contribute to sustainable energy production, yet H2S may reduce the process efficiency and gas quality. This work examined the impact of H2S on biomethanation in batch assays and in continuous trickle bed reactor (TBR). The batch assay (not biofilm based) was conducted to quickly determine the threshold H2S concentration and to evaluate the inoculum's response to repeated H2S exposure. In contrast, the TBR experiment aimed to explore the role of biofilm-based biomethanation in mitigating H2S inhibition. Batch assays revealed significant inhibition, especially at higher H2S concentrations (3 %) and thermophilic temperatures (51 °C). In the batch assay, presence of H2S resulted in up to 30 % reduction in CH4 yield, decreasing from 229 to 160 NmL/Lreactor. Additionally, the CH4 content declined by 12 %, from 49 to 43 %. In contrast, TBRs showed resilience where TBRs fed with H2S-rich biogas produced effluent gas with 83.5 % CH4, similar to control (81.0 %). 16S rRNA analysis highlighted shifts toward sulphate reducing and sulphur oxidizing bacteria under H2S exposure, while acetogenic and syntrophic acetate-oxidizing bacteria increased in the control. This suggests potential competition for available substrates when subjected to H2S. These findings highlight that H2S significantly inhibits non-biofilm-based biomethanation, as seen in batch assays, although moderate acclimation was observed. However, biofilm-based process, e.g TBRs, effectively mitigate H2S toxicity, ensuring efficient biogas upgrading to biomethane.
Forfattere
Getachew Birhanu Abera Aryan Bhusal Thea Os Andersen Shuai Wang Nabin Aryal Svein Jarle Horn Lu FengSammendrag
In-situ biomethanation is an efficient process for converting carbon dioxide (CO2) to methane (CH4) using hydrogen (H2) alongside anaerobic digestion (AD) process. However, AD of protein rich substrate often leads to the accumulation of ammonia nitrogen at high concentration. As a major inhibitor, this accumulation affects not only the AD process but also in-situ biomethanation. This study investigated the impact of ammonia nitrogen (0.5–5 g/L) on biomethanation performance using anaerobic moving-bed biofilm reactors (AnMBBRs). Without biofilm/biocarrier support, methane production was significantly inhibited above 3 g/L of ammonia nitrogen. In contrast, AnMBBR maintained high methane yields of 156.5 NmL/Lreactor at 2.5 g/L and 151.3 NmL/Lreactor at 5 g/L ammonia nitrogen, representing increases of 49 % and 76 %, respectively, compared to reactors without biofilm. Microbial analysis via 16S rRNA sequencing showed that Methanothermobacter, a thermophilic hydrogenotrophic methanogen, increased in relative abundance under ammonia nitrogen stress, which was further supported by carbon isotope analysis. Overall, these results highlighted the potential of AnMBBR to overcome ammonia nitrogen stress in in-situ biomethanation.
Forfattere
Begüm Bilgiç Thea Os Andersen Getachew Birhanu Abera Michal Sposob Lu Feng Svein Jarle HornSammendrag
Syngas biomethanation represents a promising pathway to convert recalcitrant feedstocks into biomethane. However, the hydrogen (H2) content in syngas is often insufficient or fluctuates, which affects the overall performance. This study evaluated the effect of H2 addition on syngas conversion efficiency and microbial community dynamics using two trickle bed reactors (TBRs). One TBR was fed with syngas, while another received syngas supplemented with H2. Both TBRs demonstrated the feasibility of converting CO from syngas to methane, with the H2 supplemented TBR outperforming the syngas-only TBR. The H2 supplemented TBR achieved over 90 % conversion rate at a gas loading rate of 15 NL/Lreactor/d and reached peak methane production at a gas loading rate at 20 NL/Lreactor/d. Microbial community structure analysis revealed a dominance of Methanobacterium, a known thermophilic hydrogenotrophic methanogen. Although H2 addition enhanced performance, a decline in conversion efficiency at higher gas loading rates highlights the need for further optimization.
Forfattere
Getachew Birhanu Abera Erik Trømborg Linn Solli Juline M Walter Radziah Wahid Espen Govasmark Svein Jarle Horn Nabin Aryal Lu FengSammendrag
Det er ikke registrert sammendrag
Forfattere
Michal SposobSammendrag
This study aimed to evaluate and optimize trickle bed reactor (TBR) performance for biological biogas upgrading at different gas loading rates (10-35 m3/m3d) by adjusting H2 flow (H2/CO2 ratio 4-3.7) and utilizing various packing materials. The three TBR reactors operated at thermophilic conditions (50○C) with different packing materials under same gas loading rate. Obtained results indicated that optimal performance was achieved at a gas loading rate of 14.3 m3/m3d and H2/CO2 ratio of 3.7, with average CH4 concentrations in the effluent gas from 90.8 % to 91.5 %, regardless of the packing material employed. Increasing the gas loading rate resulted in decreased CH4 content (<90 %), indicating limited treatment capacity at higher loading rates. The studied packing materials had a slight impact on reactor performance indicating that the shape of the making material has a greater influence of the reactor performance. Microbial communities analyses revealed dominance of hydrogenotrophic methanogens (Methanobacterium, Methanothermobacter, and Methanoculleus). This study highlights the importance of optimizing the H2/CO2 and considering packing materials for TBR performance.
Sammendrag
Det er ikke registrert sammendrag
