Thin biofilm can transform CO₂ into renewable energy
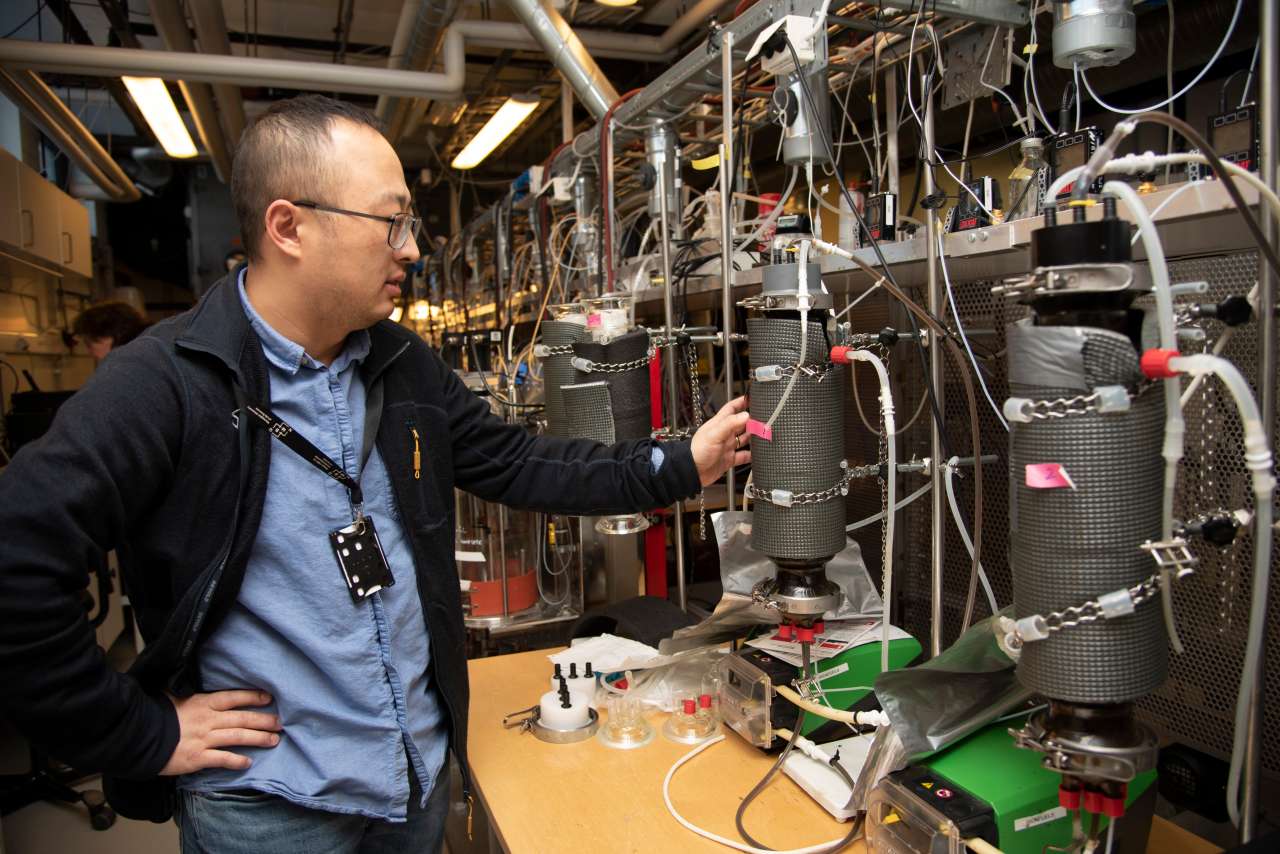
NIBIO-researcher Lu Feng and colleagues from NIBIO and NMBU have documented how biofilm-based processes can be used to produce biomethane with over 96 percent purity. Photo: John Olav Oldertrøen
NIBIO has contributed to developing a method for turning greenhouse gases like CO₂ or CO into biomethane – a renewable energy source. Using thin layers of microorganisms, so-called biofilms, greenhouse gases can be transformed into clean-burning fuel.
Carbon-based gases such as carbon dioxide (CO₂) and carbon monoxide (CO) are often associated with pollution and climate change. But what if these gases could be turned into something useful instead – like clean-burning fuel?
This is what Dr Lu Feng and other researchers have been working on. The goal of the collaboration has been to develop a new method for producing green biomethane, a sustainable alternative to natural gas.
Through five scientific papers, the researchers have documented how biofilm-based processes can be used to produce biomethane with over 96 percent purity.
Engineered biofilm for targeted conversion
A biofilm is a layer of microorganisms that grow on surfaces. The microbes work together and form a kind of community that can process gases and turn them into methane.
“Instead of decomposing organic waste, as is done in traditional biogas production, the biofilm method captures and processes gas streams using self-selected microorganisms harbored within thin biofilm under oxygen free conditions,” Dr Feng explains.
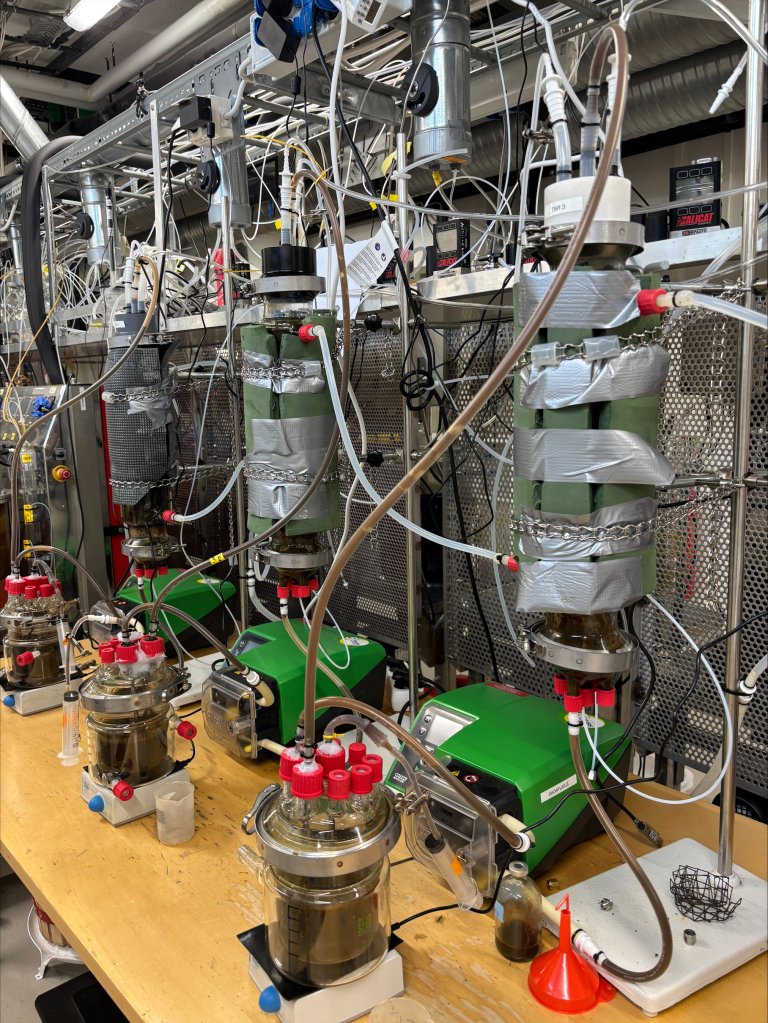
“Biofilms are widespread in nature,” he continues. “Our aim has been to engineer the biofilm to work for us for targeted conversion, either by using fixed or moving bed reactors. This opens new opportunities to convert climate-impacting gases into valuable energy.”
Among other things, the researchers experimented with adding selected microorganisms – a process known as bioaugmentation – to improve methane production.
“By introducing specific methane-producing microbes into the reactors, we were able to steer the process towards more efficient CO₂ conversion,” says Dr Feng.

Biofilm reactors maintain high methane quality and tolerance
The researcher says that the biofilms they have developed provide a stable and efficient process.
“They help retain the microbes, improve gas–liquid contact, and largely increase contact surface for the reaction. They also tolerate harmful substances that would otherwise disrupt gas production.”
In particular, biofilms can help manage challenges such as high levels of ammonia and hydrogen sulphide (H2S). These are substances often found in industrial gas streams and can be problematic in conventional bioreactors.
“In one of our studies, we tested how biofilm reactors handle H2S which is a toxic gas that can significantly reduce methane production,” says Dr Feng.
“The results showed that systems without biofilm lost up to 30 percent of the methane, while the biofilm reactors maintained high methane quality even at extremely high H2S content.”
The researchers also examined the effect of ammonia, which usually inhibits methane production. In this study, they used a type of reactor called AnMBBR (Anaerobic Moving Bed Biofilm Reactor), and found that the biofilms were able to produce methane even at high ammonia concentrations.
“High ammonia can be accumulated when fish sludge, animal slurry, or food waste are used to produce biogas”, says Dr Feng.
“Our analysis showed that the biofilm contained microbes that tolerate ammonia, including a group called Methanothermobacter, which can use H₂ and CO₂ to produce methane.”
Unlocks great potential from unconventional substrates
In another study, the researchers tested the biofilm method on syngas – a combination of hydrogen and carbon monoxide.
“This could unlock the potential of using waste to produce biomethane, for example plastic waste and woody biomass, which under normal circumstances does not degrade in a bioprocess,” Dr Feng says.
The researchers found that adding extra hydrogen could increase methane production.
Too much hydrogen, however, led to imbalance in the process.
“This shows that biofilm reactors have great potential, but also that they require careful control to function optimally at industrial scale,” says Dr Feng.
“Biofilm-based processes offer a robust and flexible platform for future biogas production. This could become an important contribution to reducing harmful gas emissions while producing renewable energy,” he adds.
Contacts

Lu Feng
Research Scientist
-
Division of Environment and Natural Resources
(+47) 458 35 202 lu.feng@nibio.no Office Location: Ås Vollebekk
The biofilm research collaboration
These works are outcomes of a close collaboration between the Norwegian Institute of Bioeconomy Research (NIBIO) and the Norwegian University of Life Sciences. Together, the researchers have developed a method that can help turn carbon emissions into a resource – while also supporting national and EU goals for a more circular and climate-friendly economy.
Five scientific papers have been produced in 2024 and 2025. Links to these are included below.
What is a biofilm?
A biofilm is a syntrophic community of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric combination of extracellular polysaccharides, proteins, lipids and DNA. Because they have a three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".
Biofilms may form on living (biotic) or non-living (abiotic) surfaces and can be common in natural, industrial, and hospital settings. They may constitute a microbiome or be a portion of it.
Source: Wikipedia
Biofilm under aerobic and anaerobic conditions
Biofilms can develop under both aerobic (with oxygen) and anaerobic (without oxygen) conditions.
In aerobic biofilms, such as those found in wastewater treatment plants or on wet surfaces exposed to air, oxygen is a key driver of microbial metabolism. These biofilms often include nitrifying bacteria or aerobic decomposers.
In anaerobic biofilms, such as those found in digesters, sediments, or the gastrointestinal tract, oxygen is absent. Here, anaerobic bacteria and archaea carry out fermentation, acidogenesis, and methanogenesis—steps critical for biogas production and carbon recycling.
Contacts

Lu Feng
Research Scientist
-
Division of Environment and Natural Resources
(+47) 458 35 202 lu.feng@nibio.no Office Location: Ås Vollebekk
Publications
Abstract
Upgrading biogas to biomethane could contribute to sustainable energy production, yet H2S may reduce the process efficiency and gas quality. This work examined the impact of H2S on biomethanation in batch assays and in continuous trickle bed reactor (TBR). The batch assay (not biofilm based) was conducted to quickly determine the threshold H2S concentration and to evaluate the inoculum's response to repeated H2S exposure. In contrast, the TBR experiment aimed to explore the role of biofilm-based biomethanation in mitigating H2S inhibition. Batch assays revealed significant inhibition, especially at higher H2S concentrations (3 %) and thermophilic temperatures (51 °C). In the batch assay, presence of H2S resulted in up to 30 % reduction in CH4 yield, decreasing from 229 to 160 NmL/Lreactor. Additionally, the CH4 content declined by 12 %, from 49 to 43 %. In contrast, TBRs showed resilience where TBRs fed with H2S-rich biogas produced effluent gas with 83.5 % CH4, similar to control (81.0 %). 16S rRNA analysis highlighted shifts toward sulphate reducing and sulphur oxidizing bacteria under H2S exposure, while acetogenic and syntrophic acetate-oxidizing bacteria increased in the control. This suggests potential competition for available substrates when subjected to H2S. These findings highlight that H2S significantly inhibits non-biofilm-based biomethanation, as seen in batch assays, although moderate acclimation was observed. However, biofilm-based process, e.g TBRs, effectively mitigate H2S toxicity, ensuring efficient biogas upgrading to biomethane.
Authors
Getachew Birhanu Abera Aryan Bhusal Thea Os Andersen Shuai Wang Nabin Aryal Svein Jarle Horn Lu FengAbstract
In-situ biomethanation is an efficient process for converting carbon dioxide (CO2) to methane (CH4) using hydrogen (H2) alongside anaerobic digestion (AD) process. However, AD of protein rich substrate often leads to the accumulation of ammonia nitrogen at high concentration. As a major inhibitor, this accumulation affects not only the AD process but also in-situ biomethanation. This study investigated the impact of ammonia nitrogen (0.5–5 g/L) on biomethanation performance using anaerobic moving-bed biofilm reactors (AnMBBRs). Without biofilm/biocarrier support, methane production was significantly inhibited above 3 g/L of ammonia nitrogen. In contrast, AnMBBR maintained high methane yields of 156.5 NmL/Lreactor at 2.5 g/L and 151.3 NmL/Lreactor at 5 g/L ammonia nitrogen, representing increases of 49 % and 76 %, respectively, compared to reactors without biofilm. Microbial analysis via 16S rRNA sequencing showed that Methanothermobacter, a thermophilic hydrogenotrophic methanogen, increased in relative abundance under ammonia nitrogen stress, which was further supported by carbon isotope analysis. Overall, these results highlighted the potential of AnMBBR to overcome ammonia nitrogen stress in in-situ biomethanation.
Abstract
Syngas biomethanation represents a promising pathway to convert recalcitrant feedstocks into biomethane. However, the hydrogen (H2) content in syngas is often insufficient or fluctuates, which affects the overall performance. This study evaluated the effect of H2 addition on syngas conversion efficiency and microbial community dynamics using two trickle bed reactors (TBRs). One TBR was fed with syngas, while another received syngas supplemented with H2. Both TBRs demonstrated the feasibility of converting CO from syngas to methane, with the H2 supplemented TBR outperforming the syngas-only TBR. The H2 supplemented TBR achieved over 90 % conversion rate at a gas loading rate of 15 NL/Lreactor/d and reached peak methane production at a gas loading rate at 20 NL/Lreactor/d. Microbial community structure analysis revealed a dominance of Methanobacterium, a known thermophilic hydrogenotrophic methanogen. Although H2 addition enhanced performance, a decline in conversion efficiency at higher gas loading rates highlights the need for further optimization.
Abstract
No abstract has been registered
Authors
Getachew Birhanu Abera Erik Trømborg Linn Solli Juline M Walter Radziah Wahid Espen Govasmark Svein Jarle Horn Nabin Aryal Lu FengAbstract
No abstract has been registered